Osmosis and diffusion are both types of transports. Diffusion is the process of which molecules spread from areas of high concentration, to areas of low concentration. Osmosis is the diffusion of water through a membrane, moving molecules from an area of high concentration, to an area of low concentration The diffusion of water molecules across the cell membrane is called osmosis. Water is isotonic and moves freely across the cell membrane and helps maintain its Lab Report Title Diffusion and Osmosis Through Nonliving Membranes Introduction Diffusion is the movement of a substance from an area of high concentration to an area of lower concentration. Diffusion occurs in liquids and gases when their particles collide randomly and spread out. Diffusion is an important process for living things, it is how
AP Lab 1: Osmosis and Diffusion Lab Report - Allysha's e-Portfolio
Statement of the Problem:. How does diffusion across the cell membrane work? What molecules pass through the cell membrane easier than others? Is there any influence of solute concentration to the net movement of water molecules across the cell membrane?
What is the effect of water potential on the cell membrane? Background Information:. Cell membranes act as a barrier for the cell. It keeps together enzymes, DNA, and pathways for metabolic reactions, osmosis and diffusion lab report. Cell membranes dispose of waste products from the cell and lets important molecules, like water and oxygen, into the cell.
The membrane is semipermeable, meaning only specific osmosis and diffusion lab report may enter the cell. The passing of molecules is either through active transport passage of materials using energy or passive transport passage of materials using kinetic energy.
Molecules are in constant, random motion Brownian motion and if they collide with the membrane, they will rebound. If the molecules are headed toward an open pore in the cell membrane, it may pass through the pore or rebound depending on its size to the pore. The passage of molecules across the cell membrane from an area of high concentration to low concentration is call diffusion.
The diffusion of water molecules across the cell membrane is called osmosis. Water is isotonic and moves freely across the cell membrane and helps maintain its fluid mosaic model characteristic. Hypertonic solutions are solutions with higher amounts of solutes and hypotonic solutions are solutions with a lower amount of solutes. The movement of water across the cell membrane depends on the concentration of solutes on both sides of the cell membrane.
When water moves out of the cell, the cell will shrink, and when water moves into the cell, the cell will swell and possibly burst. Cell walls are present in plant cells which prevent osmosis and diffusion lab report cell from bursting once it swells. When water enters the plant cell, the membrane is pressed up against the cell wall and creates turgor pressure. Water potential is used to sum up the differences in solute concentration and pressure to predict the direction water will diffuse in living plant tissues, osmosis and diffusion lab report.
Water potential is measured in bars, metric units of pressure equal to 10 newtons per cm 2 or 1 atmosphere. Two major factors of water potential are solute potential Ψsthe dependent on solute concentration, and pressure potential Ψpwhich represents exertion of pressure on a solution in positive or negative.
Pure water has a water potential of 1 atmosphere. Dissolving substances in water will result the water potential dropping below zero. When solute concentration increases, water potential decreases. Pressure potential may be positive, negative, or zero. Even though water is diffused in all directions, water will always diffuse from an area of osmosis and diffusion lab report water potential to and area of low water potential.
A value of -9 will be assigned for variable Ψs. The process of the cell wall pulling away from the cell membrane in a plant cell is called plasmolysis. If we determine the molarity of the sucrose solution that will help produce equilibrium between the solution and the contents of the potato cell, we can determine solute potential: Ψs — iCRT, where i is ionization constant, C is molar concentration of sucrose per liter at equilibrium, R is pressure constant 0.
Activity A: Diffusion. Hypothesis: If we add glucose-starch solution to a dialysis tubing bag and submerged it in a cup of distilled water and IKI solution, then glucose will leave the dialysis tubing bag through pores into the IKI solution through diffusion. Dropping Pipet. First, we poured mL of distilled water into a cup and added about 4 mL of IKI solution to the water and mixed well. We recorded the initial color of the solution in Table 1.
After we were finished, we discarded the used glucose test strip. We recorded the initial glucose test result in Table 1. Then we discarded the used glucose test strip. After soaking a piece of dialysis tubing in water, a group member rolled the tubing between their thumb and index finger to open it. We tied one end of the dialysis tubing to create a bag.
Then we tied off the top of the bag to close it while leaving enough room in the bag for expansion, osmosis and diffusion lab report.
We then placed the dialysis bag into the solution in the cup. In doing so, we made sure the entire bag was covered by the solution in the cup, osmosis and diffusion lab report. We then waited 30 minutes and worked on an activity relating to Figure 2. Figure 2 Activity:.
We were to indicate initial locations of molecules and predict in which direction they would move in diffusion into the bag, out of the bag, both into and out of the bag, or none. After completing the activity with figure 2, we were able to compare our predictions about the outcome to the actual results of the experiment. Distilled water was initially in the cup and is predicted to stay in the cup.
IKI was initially in the cup and is predicted to stay in the cup and also move into the dialysis bag. Glucose was initially in osmosis and diffusion lab report dialysis bag and is predicted to flow in and out of the dialysis bag and exist in both the cup and dialysis bag. Sucrose was initially in the dialysis bag and is predicted to stay in the dialysis bag. After 30 minutes, we removed the dialysis bag from the cup and dried it with a paper osmosis and diffusion lab report. We then cut a hole into the bag large enough for a glucose test strip to enter.
We then collected the final amounts of glucose and completed Table 1. Table 1. Solution Color. Glucose Test Results. Dialysis Bag. Brownish Red. When comparing our results to our predictions, osmosis and diffusion lab report, we had predicted correctly.
We had no conflicts that would have made us revise our predictions. The results show the locations each molecule had gone and were the molecules ended up and proved our predictions were correct.
This activity proved the net movement of glucose from the dialysis bag to the cup and both the cup and dialysis tested positive for glucose at the end of the experiment. The data in this experiment tells us the sizes of molecules would have to be small enough to fit through the dialysis tubing because if it is not small enough, then the tubing will rebound the molecules and not let them pass through.
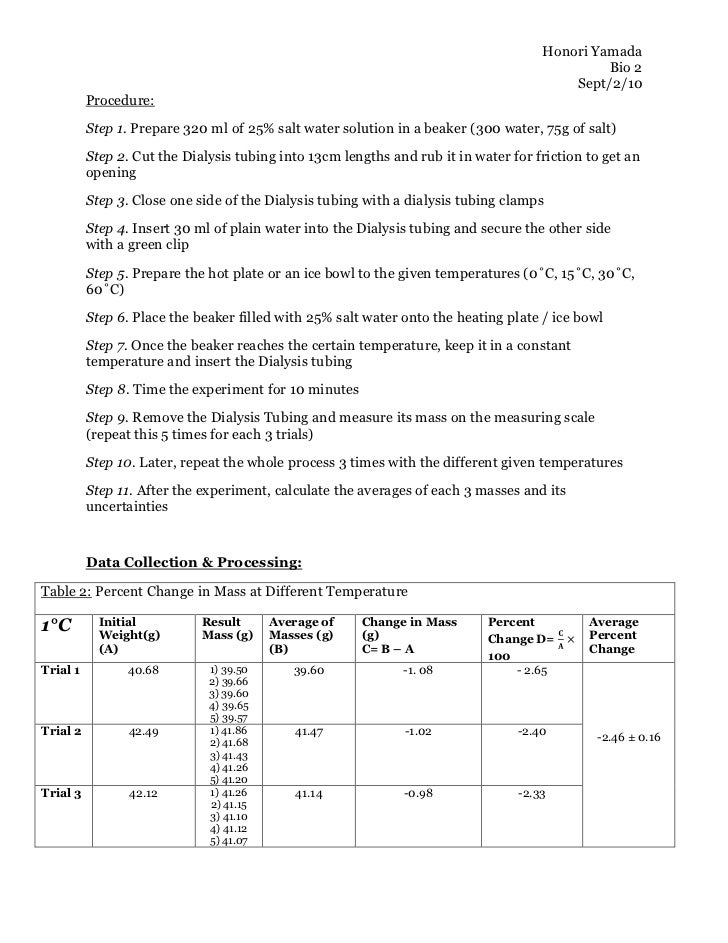
Activity B: Osmosis. Hypothesis: If we add higher concentrations of sucrose to the dialysis bag, then the net movement of water into the dialysis bag will increase. Paper Towels. Electronic Balance. For each sucrose solution, we poured mL of distilled water into a cup. We then labeled the cup with the concentration of sucrose that we tested. A group member then took a piece of dialysis tubing and opened it by rolling it between their thumb and index finger after being soaked in water.
Then we tied off one end of the dialysis tubing to create a bag. Using a funnel, we poured 25 mL of sucrose solution into the dialysis bag. Leaving enough room in the bag for expansion, we tied off the bag and dried it will paper towels, osmosis and diffusion lab report. We weighed the bag and recorded its initial mass in Table 2. After measuring osmosis and diffusion lab report mass, we placed the bag in the cup of water, making sure the bag was completely submerged in the water.
We waited for 30 minutes before continuing. After 30 minutes, we removed the bag from the water and dried it with paper towels. We then weighed the bag and recorded the final mass in Table 2.
Table 2. Contents in the Dialysis Bag. Initial Mass. Final Mass. Change in Mass. Percent Change in Mass.
To calculate the percent change in mass, we used the formula:. The change in mass in this activity indicates whether or not a solution entered or left the dialysis bags during the experiment. In the case of Activity B, the change in mass increased and shows water entered the bags during the experiment. In this experiment, the variable being tested is water.
Osmosis is the diffusion of water from osmosis and diffusion lab report high concentration to a low concentration and water was the variable being tested in this activity because it is what made the mass increase for every sucrose solution.
The amount of sucrose solution, dialysis bag, and time could all influence the outcome of this experiment. The higher the amount of sucrose causes for more water to move into the dialysis bag. The dialysis bag is an influence because of pore size in relation to the size of the water molecules, osmosis and diffusion lab report. Time could influence the movement of water because osmosis and diffusion lab report, with more time, more water will move into the dialysis bag than shorter time periods.
The graph we made is an accurate representation of our data and how the mass changed due to the sucrose solutions because water was adding to the weight of the dialysis bag. In osmosis, water molecules moved into the dialysis bags with higher osmosis and diffusion lab report molarities.
The solutions in the bag and outside of the bag were not isotonic to each other during this experiment because of the change in mass.
osmosis and diffusion lab report video
, time: 2:29Lab Report On Diffusion And Osmosis | blogger.com
Oct 12, · This lab, title Diffusion and Osmosis, was centered around the diffusion across a cellular membrane and how exactly materials move and diffuse in concentrations. Both diffusion and osmosis are Osmosis and diffusion are both types of transports. Diffusion is the process of which molecules spread from areas of high concentration, to areas of low concentration. Osmosis is the diffusion of water through a membrane, moving molecules from an area of high concentration, to an area of low concentration Lab Report Title Diffusion and Osmosis Through Nonliving Membranes Introduction Diffusion is the movement of a substance from an area of high concentration to an area of lower concentration. Diffusion occurs in liquids and gases when their particles collide randomly and spread out. Diffusion is an important process for living things, it is how
No comments:
Post a Comment